Environmental and Biomedical Applications of Natural Metal Stable Isotope Variations
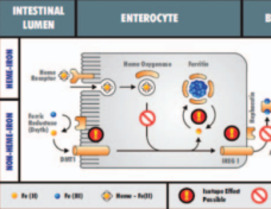
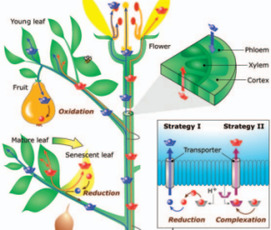
Metal stable isotopes are now being used to trace metal contaminants in the environment and as indicators of human systemic function where metals play a role. Stable isotope abundance variations provide information about metal sources and the processes affecting metals in complex natural systems, complementing information gained from surrogate tracers, such as metal abundance ratios or biochemical markers of metal metabolism. The science is still in its infancy, but the results of initial studies confirm that metal stable isotopes can provide a powerful tool for forensic and biomedical investigations.
Environmental and Biomedical Applications of Natural Metal Stable Isotope Variations Read More »