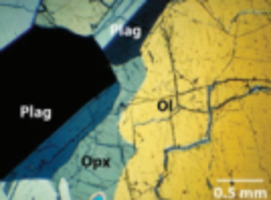
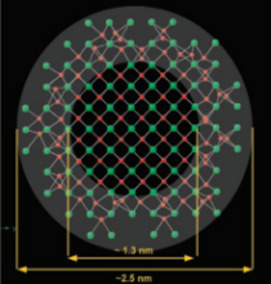
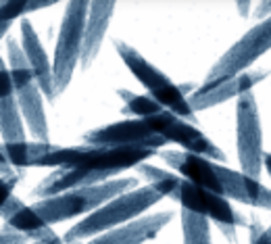
Soils contain many kinds of inorganic and organic particles with at least one dimension in the nanoscale or colloidal range (<100 nm). Well-known examples are clay minerals, metal (hydr)oxides, and humic substances, while allophane and imogolite are abundant in volcanic soils. Apparently, only a small proportion of nanoparticles in soil occur as discrete entities. Organic colloids in soil, for example, are largely associated with their inorganic counterparts or form coatings over mineral surfaces. For this reason, individual nanoparticles are difficult to separate and collect from the bulk soil, and extraction yields are generally low. By the same token, the characterization of soil nanoparticles often requires advanced analytical and spectroscopic techniques. Because of their large surface area and the presence of surface defects and dislocations, nanoparticles in soil are very reactive towards external solute molecules. The focus of research in recent years has been on the interactions of nanoparticles with environmental pollutants and on their impact on the movement, fate, and bioavailability of contaminants.><100 nm). Well-known examples are clay minerals, metal (hydr) oxides, and humic substances, while allophane and imogolite are abundant in volcanic soils. Apparently, only a small proportion of nanoparticles in soil occur as discrete entities. Organic colloids in soil, for example, are largely associated with their inorganic counterparts or form coatings over mineral surfaces. For this reason, individual nanoparticles are difficult to separate and collect from the bulk soil, and extraction yields are generally low. By the same token, the characterization of soil nanoparticles often requires advanced analytical and spectroscopic techniques. Because of their large surface area and the presence of surface defects and dislocations, nanoparticles in soil are very reactive towards external solute molecules. The focus of research in recent years has been on the interactions of nanoparticles with environmental pollutants and on their impact on the movement, fate, and bioavailability of contaminants.