CO2 Sequestration in Deep Sedimentary Formations
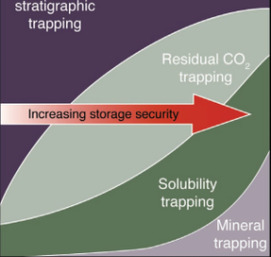
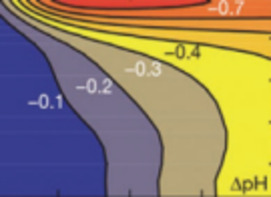
Carbon dioxide capture and sequestration (CCS) in deep geological formations has recently emerged as an important option for reducing greenhouse emissions. If CCS is implemented on the scale needed to make noticeable reductions in atmospheric CO2, a billion metric tons or more must be sequestered annually—a 250 fold increase over the amount sequestered today. Securing such a large volume will require a solid scientific foundation defining the coupled hydrologic–geochemical–geomechanical processes that govern the long-term fate of CO2 in the subsurface. Also needed are methods to characterize and select sequestration sites, subsurface engineering to optimize performance and cost, approaches to ensure safe operation, monitoring technology, remediation methods, regulatory overview, and an institutional approach for managing long-term liability.
CO2 Sequestration in Deep Sedimentary Formations Read More »