Shining Light on Metals in the Environment
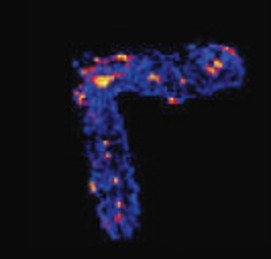
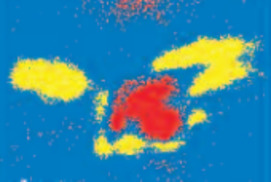
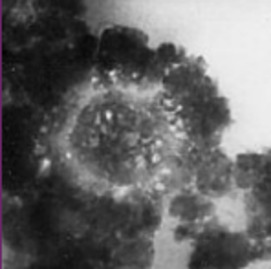
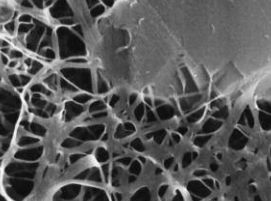
Elucidating the speciation of heavy metals in the environment is para- mount to understanding their potential mobility and bioavailability. Cutting-edge synchrotron-based techniques such as microfocused X-ray absorption fine-structure (XAFS) and X-ray fluorescence (XRF) spectroscopy and microtomography have revolutionized the way metal reactions and processes in natural systems are studied. In this article, we apply these intense-light tools to decipher metal forms (species) and associations in contaminated soils and metal-hyperaccumulating plants.
Shining Light on Metals in the Environment Read More »