Metal Stable Isotopes: Signals in the Environment
Thomas D. Bullen and Anton Eisenhauer – Guest Editors
Table of Contents
During the past decade it has been recognized that the stable isotope compositions of several metallic elements vary significantly in nature due to both biotic and abiotic processing. While this leap in our understanding has been fueled by recent advances in instrumentation and techniques in both thermal ionization and inductively coupled plasma mass spectrometry, the field of metal stable isotope geochemistry has finally moved beyond a focus on development of analytical techniques and toward using the isotopes as source and process tracers in natural and experimental systems. Often termed the “nontraditional stable isotopes,” metal stable isotope systems have found wide application in the geological, hydrological, and environmental research realms and are enjoying a rapidly expanding presence in the scientific literature. This issue of Elements will focus on several intriguing aspects of lowtemperature metal stable isotope geochemistry.
Metal Stable Isotopes in Low-Temperature Systems: A Primer
The Odds and Evens of Mercury Isotopes: Applications of Mass-Dependent and Mass-Independent Isotope Fractionation
Reconstructing Paleoredox Conditions through a Multitracer Approach: The Key to the Past Is the Present
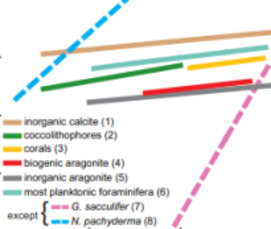
Marine Calcification: An Alkali Earth Metal Isotope Perspective
Combining Metal Stable Isotope Fractionation Theory with Experiments
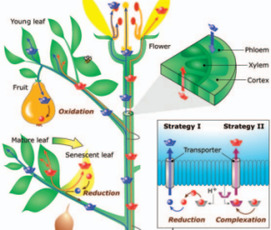
Fractionation of Metal Stable Isotopes by Higher Plants
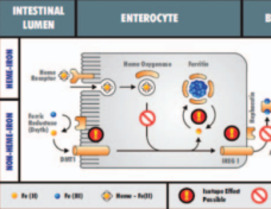
Environmental and Biomedical Applications of Natural Metal Stable Isotope Variations
Activation Laboratories (Actlabs)
Cambridge University Press
Excalibur
GeoScienceWorld
IMA 2010
Nu Instruments
Rocks & Minerals
RockWare
Savillex
Smart Elements
Spectromat
Thermo
v6n1 Mineral Evolution
Guest editor: Robert M. Hazen (Geophysical Laboratory)
“Mineral evolution,” the study of Earth’s changing near-surface mineralogy, frames Earth materials research with a historical narrative. This 4.5-billion-year story integrates themes of planetary science, including geodynamics, petrology, geo- chemistry, thermodynamics, geobiology, and more. Mineralogy thus holds the key to unlocking our planet’s history and assumes its rightful central role in the Earth sciences. The mineralogy of terrestrial planets evolves as a consequence of physical, chemical, and biological pro- cesses. Starting with ~12 refractory min- erals in prestellar molecular clouds, processes in the solar nebula led to the ~250 different minerals found in mete- orites. Initial mineral evolution of Earth’s crust depended on a sequence of geochemical and petrologic processes that resulted in an estimated 1500 dif- ferent mineral species. Ultimately, biological processes produced large-scale changes in atmospheric and ocean chemistry that may be responsible, directly or indirectly, for most of Earth’s 4400 known mineral species. Mineral evolution thus highlights the coevolution of the geo- and biospheres.
- Mineral Evolution: Mineralogy in the Fourth Dimension – Robert M. Hazen (Geophysical Laboratory) and John M. Ferry (Johns Hopkins University)
- Themes and Variations in Complex Systems – Robert M. Hazen (Geophysical Laboratory) and Niles Eldredge (American Museum of Natural History)
- Mineralogical Evolution of Meteorites – Timothy J. McCoy (Smithsonian Institution)
- Mineral Environments on the Earliest Earth – Dominic Papineau (Geophysical Laboratory)
- The Evolution of Elements and Isotopes – Hendrik Schatz (Michigan State University)
- The Great Oxidation Event and Mineral Diversification – Dimitri A. Sverjensky and Namhey Lee (Johns Hopkins University)
- The Rise of Skeletal Biominerals – Patricia M. Dove (Virginia Tech)
- Scientific Exploration of the Moon (February 2009)
- Bentonites – Versatile Clays (April 2009)
- Gems (June 2009)
- Mineral Magmatism: From Microbes to Meteorites (August 2009)
- Gold (October 2009)
- Metal Stable Isotopes: Signals in the Environment (December 2009)
Download 2009 Thematic Preview
- Mineral Evolution (February 2010)
- Sulfur (April 2010)
- Fluids in Metamorphism (June 2010)
- Atmospheric Particles (August 2010)
- Thermodynamics of Earth Systems (October 2010)
- Sustainable Remediation of Soil (December 2010)
Download 2010 Thematic Preview