Boron: Light and Lively
Edward S. Grew – Guest Editor
Table of Contents
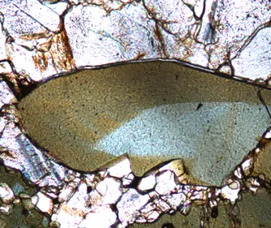
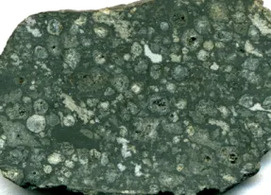
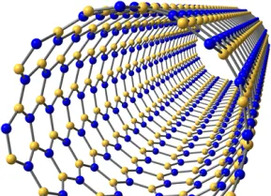
Fifth in the periodic table, boron is a “light” element whose origin has puzzled astronomers because it is not created in stars. It is “lively”, being an essential element for plants, and having medicinal properties, which has stimulated synthesis of organic compounds containing boron. Borates such as colemanite are thought by some to have stabilized ribose, an essential component of ribonucleic acid and critical for the self-assembly of prebiotic organic compounds to constitute life; others have proposed that ribose was stabilized by borate in solution. Boron isotopes provide insight on the processes responsible for the creation of continental crust, and act as a proxy for paleoclimate. Extreme concentrations of boron result in economic evaporitic deposits, and, thus, water-soluble boron minerals, notably borax, have been among the most accessible of useful compounds to humankind, even in antiquity.
Analab
Australian Scientific Instruments (ASI)
CAMECA
Crystal Maker
Elemental Scientific, Inc.
Excalibur Mineral Corporation
International Mineralogical Association
IsotopX
PANalytical
ProtoXRD
Savillex
Selfrag
Volume 13, Number 5 (October) • Mineral Resources and Sustainable Development
GUEST EDITOR: Georges Calas
Mineral resources are a vital part of any economy, modern or ancient. Since the birth of civilization, man has used these resources for pigments, metals, glasses, ceramics, cements and much more. The media has recently suggested there is a crisis looming over finding mineral resources, including critical metals. Centered on the sustainability of mineral resources, themes addressed in this issue include customer–supplier relationships, exploration, recycling and the circular economy, and environmental post-mining impacts. The broad range of topics embraced by this issue – formation of mineral deposits, minerals engineering, and environmental and societal impacts – will provide readers a better understanding of the large-scale economic, historical and educational aspects of mineral resources.
- How Volcanoes Work (February 2017)
- Sulfides (April 2017)
- Rock and Mineral Coatings: Records of Climate Change, Pollution, and Life (June 2017)
- Boron: Light and Lively (August 2017)
- Mineral Resources and Sustainable Development (October 2017)
- Layered Intrusions: Natural Laboratories for Magma Chamber Processes (December 2017)