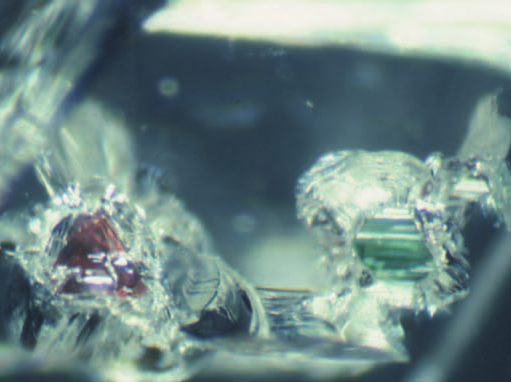
Diamonds
George E. Harlow, and Rondi M. Davies – Guest Editors
Table of Contents
Diamond, the fascinating ultrahard mineral, is the focus of considerable interest and scientific research. Recent advances particularly relevant to geoscientists include: diamond as a recorder of Earth processes from the perspective of inclusions, chemistry, and conditions of formation; syn- thesis for research applications and processing to modify color and physical properties, important to diamond gems and anvils; the implications of nanodia- monds from meteorites.
- Diamonds
- Inclusions in Sublithospheric Diamonds: Glimpses of Deep Earth
- Stable Isotopes and the Origin of Diamond
- Strange Diamonds: The Mysterious Origins of Carbonado and Framesite
- Microdiamonds in Ultrahigh-Pressure Metamorphic Rocks
- Meteoritic Nanodiamonds: Messengers from the Stars
- High-Pressure High Temperature Treatment of Gem Diamonds
- Growing Diamond Crystals by Chemical Vapor Deposition
Environmental Isotope Laboratory
Excalibur Mineral Corporation
Gemological Institute of America
HORIBA Jobin Yvon
Hudson Institute of Mineralogy
Meiji Techno America
Rigaku
RockWare
SGS Lakefield Research
Universität Bayreuth
v1n3 Genesis: Rocks, Minerals, and the Geochemical Origin of Life
Guest editor: Robert M. Hazen, Geophysical Laboratory
In the beginning, Earth was a blasted, lifeless planet of water, air, and rock. From these raw materials, life emerged through a sequence of geo-chemical processes, including mineral-catalyzed organic synthesis, con-centration of those molecules in mineral surfaces, and assembly of life’s essential macromolecules.
- Genesis: Rocks, Minerals, and the Geochemical Origin of Life Robert M. Hazen (Carnegie Institution of Washington’s Geophysical Laboratory)
- Geochemical Influences on Life’s Origins and Evolution Joseph V. Smith (University of Chicago, USA)
- Mineral Catalysis and Prebiotic Synthesis: Montmorillonite-Catalyzed Formation of RNA James P. Ferris (Director of the New York Center)
- Geochemical Connections to Primitive Metabolism George D. Cody (Carnegie Institution of Washington)
- Sketches for a Mineral Genetic Material A. Graham Cairns-Smith (University of Edinburgh)
- Fluids in Planetary Systems (January 2005 )
- Diamonds (March 2005)
- Genesis: Rocks, Minerals, and the Geochemical Origin of Life (June 2005)
- Toxic Metals in the Environment: The Role of Surfaces (September 2005)
- Large Igneous Provinces: Origin and Environmental Consequences (December 2005 )