Lithium: Less is More
Robert J. Bowell, Philip A.E. Pogge von Strandmann and Edward S. Grew – Guest Editors
Table of Contents
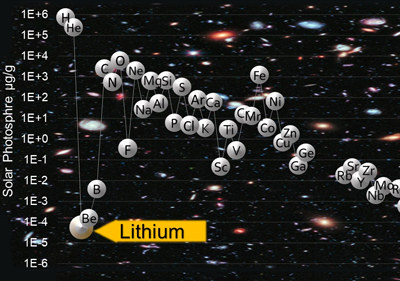
Lithium was created during the Big Bang at about 13.8 Ga. Lithium is concentrated in Earth’s upper continental crust and in 124 mineral species, the greatest mineralogical diversity being found in pegmatites. Lithium occurs naturally in two isotopes, 6Li and 7Li, which are readily fractionated, thus becoming sensitive to geological and environmental processes. Closed-basin brines (58%) and pegmatites plus related granites (26%) constitute the main sources of exploitable lithium worldwide. Life as we know it at the start of the 21st century would not be possible without lithium as it is used in a myriad of applications ranging from lithium-ion batteries to medicine.
- The Minerals of Lithium
- The Cosmic Lithium Story
- High-Temperature Processes: Is it Time for Lithium Isotopes?
- Lithium and Lithium Isotopes in Earth’s Surface Cycles
- Classification and Characteristics of Natural Lithium Resources
- From Mine to Mind and Mobiles: Society’s Increasing Dependence on Lithium
CAMECA
Crystal Maker
Excalibur Mineral Corporation
International Centre for Diffraction Data
International Association of Geoanalysts
ProtoXRD
Savillex
v16n5 Noble Gas Thermochronology
GUEST EDITORS: Marissa M. Tremblay (Purdue University, USA), Emily H.G. Cooperdock (University of Southern California, USA), and Peter K. Zeitler (Lehigh University, USA)
Noble-gas thermochronology takes advantage of two properties: (1) the time-dependent production of noble gases, such as helium and argon, by processes like radioactive decay; (2) the thermally activated diffusion of these gases to constrain the temperature histories of several minerals commonly found in crustal rocks. Because temperature is essential to many geological processes, thermochronology has become widely used to address research questions across Earth and planetary science. These questions include when and how valleys are cut by glaciers; from where is sediment sourced; what thermal conditions occur on fault planes during slip; and how the surfaces of planetary bodies evolve on billion-year timescales. This issue will highlight how noble-gas thermochronology can be used to address questions like these, as well as what new avenues of research noble-gas thermochronology could be used for in the future.
- The Thermal History of Rocks as Recorded by Noble Gases Peter K. Zeitler (Lehigh University, USA) and Cecile Gautheron (Université Paris Sud, France)
- Detrital Thermochronology: Recorder of the Earth’s Dynamic Past Daniel F. Stockli (University of Texas at Austin, USA) and Yani M.R. Najman (Lancaster University, UK; University of Colorado, Boulder, USA)
- Faults, Fluids, and Heat: New Insights from Fe-Oxide (U–Th/He) Thermochronology Emily H.G. Cooperdock (University of Southern California, USA) and Alexis K. Ault (Utah State University, USA)
- Vestiges of Deep Time: Noble-Gas Thermochronology of Ancient Rocks Kalin T. McDannell (Geological Survey of Canada, Canada) and Rebecca M. Flowers (University of Colorado, Boulder, USA)
- Noble-Gas Thermochronology of Extraterrestrial Materials Marissa M. Tremblay (Purdue University, USA) and William S. Cassata (Lawrence Livermore National Laboratory, USA)
- Lazed and Diffused: Untangling Noble-Gas Thermochronology Data Matthew Fox (University College London, UK) and David L. Shuster (University of California, Berkeley, USA)
- Abiotic Hydrogen and Hydrocarbons in Planetary Lithospheres (February 2020)
- Raman Spectroscopy in the Earth and Planetary Sciences (April 2020)
- The Redox Engine of Earth (June 2020)
- Lithium: Less is More (August 2020)
- Noble Gas Thermochronology (October 2020)
- Hydrothermal Fluids (December 2020)