Origins of Life: Transition from Geochemistry to Biogeochemistry
Nita Sahai and Hussein Kaddour – Guest Editors
Table of Contents
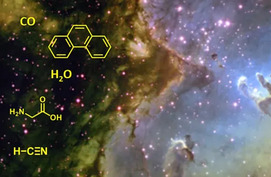
How did life arise from inorganic molecules? Did it develop in an early Earth primordial soup or was there an extraterrestrial source? Although the answer to the origin of sentient life has yet to be discovered by scientists, the origins of the genetic blueprints for life (e.g. RNA), the workhorses of life (e.g. proteins), and the protective membranes for life (e.g. lipids) are rapidly being uncovered. But, making the basic building blocks is only the first step. The next steps involve converting those molecules into viable cells. Believe it or not, geoscientists are needed to help uncover the answers to these questions because abiogenesis requires chemical, biological, and geological considerations. We hope the articles in this issue help introduce you to this exciting field of research.
Analab
Australian Scientific Instruments (ASI)
Cameca
Elemental Scientific, Inc. (ESI)
Excalibur Mineral Corporation
Geochemist’s Workbench
Gemological Institute of America
PANalytical
Periodico di Mineralogia
ProtoXRD
Rigaku
Savillex
Selfrag
Society for Geology Applied to Mineral Deposits (SGA)
Volume 13, Number 1 (February) • Volcanoes: Storage to Surface
GUEST EDITORS: Keith Putirka and Kari M. Cooper
Volcanoes have played a large role over Earth’s history in building the crust, contributing to atmospheric formation, and transferring heat and mass from the interior to the surface. They are also capable of massive disruption of the surface environment and to human civilizations. Volcanoes themselves are the products of crustal-scale systems. But what controls whether a given magma will erupt or stall, and how do processes in one part of the system affect others? Volcano science is advancing rapidly, and improvements in monitoring tools, petrologic tools, and modeling of volcanic processes have greatly improved our understanding of volcanic behavior. This issue brings together contributions exploring volcanic behavior throughout the crustal system.
- How Volcanoes Work (February 2017)
- Sulfides (April 2017)
- Rock and Mineral Coatings: Records of Climate Change, Pollution, and Life (June 2017)
- Boron: Light and Lively (August 2017)
- Mineral Resources and Sustainable Development (October 2017)
- Layered Intrusions: Natural Laboratories for Magma Chamber Processes (December 2017)
Download 2017 Thematic Preview