Phosphates and Global Sustainability
Eugenia Valsami-Jones and Eric H. Oelkers – Guest Editors
Table of Contents
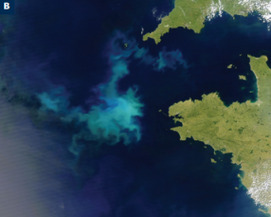
Phosphorus is a unique element: it is essential to the existence of all living forms, and as such controls biological productivity in many terrestrial and marine envi- ronments; but when in excess, it leads to uncontrollable biological growth and water-quality problems. This has become a common environmental issue, result- ing from our careless use of phosphorus in agriculture, yet phosphate ore deposits, from which fertilizers are produced, are a finite natural resource. Understanding the properties of phosphate minerals may hold the key to protecting the future of this resource. Phosphate minerals are also of extreme importance in biomineral- ization and could be the future hosts of nuclear waste. Despite all this, mineralo- gists and geochemists have invested little time understanding phosphate mineral stability, reactivity, and transformations, and this issue attempts to bring phos- phates to the forefront of our scientific endeavors.
Phosphate Mineral Reactivity and Global Sustainability
The Global Phosphorus Cycle: Past, Present, and Future
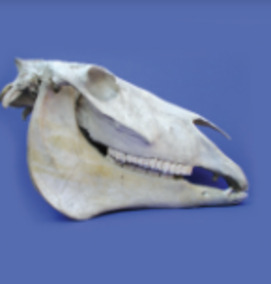
Bone and Tooth Mineralization: Why Apatite?
Phosphate Minerals, Environmental Pollution and Sustainable Agriculture
Phosphorus Removal and Recovery from Municipal Wastewaters
Phosphates and Nuclear Waste Storage
Bruker AXS
CrystalMaker
Critical Zone Exploration Network
Excalibur Mineral Corporation
Hudson Institute of Mineralogy
IWA Publishing
Meiji Techno
Rigaku
RockWare
v4n3 Deep Earth and Mineral Physics
Guest editor: Jay D. Bass (University of Illinois) and John B. Parise (Stony Brook University)
The field of high-pressure mineral physics is cen- tral to our understanding of the Earth’s interior and its evolution. It is also a field that is rapidly advancing. Recent major discoveries, such as the post-perovskite phase transition that may explain some of the properties of the core–man- tle boundary, speak to the continued impor- tance of high-pressure mineral physics experiments. The results from experimental mineral physics along with seismological data are used to construct compositional and thermal models of the Earth and its heterogeneity, including inferences of deep geochemical reservoirs. These results are also key to understanding all plane- tary bodies in the solar system. This issue of Ele- ments will highlight several key areas of high- pressure mineral physics in a form that is accessible to a broad mineralogical audience.
Deep Earth and Recent Developments in Mineral Physics Jay D. Bass (University of Illinois) and John B. Parise (Stony Brook University)
Elastic Properties of Minerals: A Key for Understanding the Composition and Temperature of Earth’s Interior Jay D. Bass (University of Illinois), Stanislav V. Sinogeikin (Carnegie Institution of Washington), and Baosheng Li (Stony Brook University)
The Upper Mantle and Transition Zone Daniel J. Frost (Bayerisches Geoinstitut)
The Earth’s Lower Mantle and Core Guillaume Fiquet, François Guyot, and James Badro (Institut de Physique du Globe de Paris, Université Pierre et Marie Curie)
Discovery of Post-Perovskite and New Views on the Core–Mantle Boundary Region Kei Hirose (Tokyo Institute of Technology) and Thorne Lay (University of California –santa Cruz)
Laboratory Studies of the Rheological Properties of Minerals under Deep-Mantle Conditions Shun-ichiro Karato and Donald J. Weidner (Stony Brook
University)
- Supervolcanoes (February 2008)
- Phosphates and Global Sustainability (April 2008)
- Deep Earth and Mineral Physics (June 2008)
- Platinum-Group Elements (August 2008)
- Carbon Dioxide Sequestration (October 2008)
- Nanogeoscience (December 2008)
Download 2008 Thematic Preview