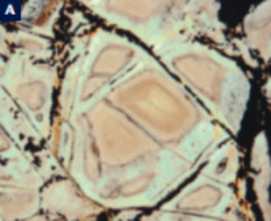
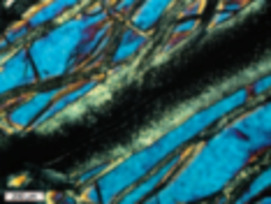
Serpentinites
Stéphane Guillot and Keiko Hattori – Guest Editors
Table of Contents
Serpentinites, primarily composed of serpentine minerals and formed by hydra- tion of peridotites, increasingly attract the attention of a wide range of scientists, including geophysicists, structural geologists, engineers, and astrobiologists. As serpentinites have wide stability fields, they form in a variety of environments, from the Earth’s surface to the interior of the mantle. They are important as reservoirs of water in the deep mantle and in the recycling of elements in sub- duction zones. Because of their physical properties, serpentinites play important roles in seismic activity and geodynamics, including earthquakes in subduction zones, rifting, oceanic spreading, strike-slip faulting, and the exhumation of deeply subducted rocks. Serpentinites are also economically important because obducted serpentinites contain more than half the world’s reserves of nickel. The formation of serpentinites is accompanied by the production of hydrogen and methane, producing unique ecosystems on the ocean floor. The generation of hydrocarbons during serpentinization is the essential fi rst step in the origin of life on Earth and possibly other planets.
- Serpentinites: Essential Roles in Geodynamics, Arc Volcanism, Sustainable Development, and the Origin of Life
- Serpentinite: What, Why, Where?
- Rheology and Tectonic Significance of Serpentinite
- Serpentinite Carbonation for CO2 Sequestration
- Nickel Laterite Ore Deposits: Weathered Serpentinites
- Serpentinites, Hydrogen, and Life
AHF Analysentechnik
Bruker
Bruker Nano
FEI
Excalibur Mineral Corporation
Geochemist’s Workbench
JEOL
McCrone Microscope and Accessories
Rigaku
Savillex
Society of Economic Geologists
SPECTRO
TSI
v9n3 THE MINERAL–WATER INTERFACE: WHERE MINERALS REACT WITH THE ENVIRONMENT
Guest Editors: Christine V. Putnis (University of Münster) and Encarnación Ruiz-Agudo (University of Granada)
Reactions occurring at mineral–water interfaces are central to geochemical pro- cesses. They affect a wide range of important environmental issues, such as the composition of natural waters, weathering and soil formation, element cycling, biomineralization (including minor-element incorporation), acid mine drainage, and nuclear waste disposal. Recent studies using state-of-the-art spectroscopic and microscopic techniques have characterized the molecular structure of mineral surfaces, the distribution of fluids near surfaces, and dynamic processes such as dissolution, growth, and mineral replacement. These studies provide insights into the kinetics and mechanisms of reactions occurring at mineral surfaces, and they test the validity of predictions based on theory. These recent advances constitute the central theme of this issue of Elements. Modeling approaches used in mecha- nistic studies are also introduced. Such approaches complement direct, in situ, molecular-scale observations of processes occurring at mineral–water interfaces.
- The Mineral–Water Interface: Where Minerals React with the Environment Christine V. Putnis (University of Münster) and Encarnación Ruiz-Agudo (University of Granada)
- A Stochastic Treatment of Crystal Dissolution Kinetics Andreas Lüttge (MARUM, University of Bremen, Rice University, Babes-Bolyai University), Rolf S. Arvidson (MARUM, University of Bremen), and Cornelius Fischer (MARUM, University of Bremen, Rice University)
- How Ions and Molecules Organize to Form Crystals H. Henry Teng (The George Washington University)
- Environmental Remediation by Crystallization of Solid Solutions Manuel Prieto (University of Oviedo), José Manuel Astilleros (Complutense University, Madrid, IGEO), and Lurdes Fernández-Díaz (Complutense University, Madrid, IGEO)
- Control of Crystal Nucleation and Growth by Additives Carlos Rodriguez-Navarro (University of Granada) and Liane G. Benning (University of Leeds)
- Virtual Probes of Mineral–Water Interfaces: The More Flops, the Better! Andrew G. Stack (Oak Ridge National Laboratory), Julian D. Gale (Curtin University), and Paolo Raiteri (Curtin University)
- One Hundred Years of Geochronology (February 2013)
- Serpentinites (April 2013)
- The Mineral-Water Interface (June)
- Continental Crust at Mantle Depths (August 2013)
- Nitrogen and Its (Biogeocosmo)Chemical Cycling (October 2013)
- Garnet: Common Mineral, Uncommonly Useful (December 2013)
Download 2013 Thematic Preview