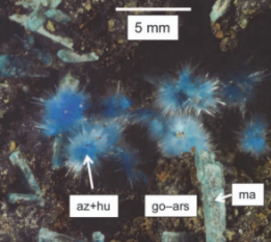
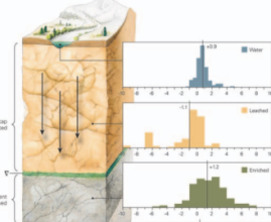
Supergene Metal Deposits
Martin Reich and Paulo M. Vasconcelos – Guest Editors
Table of Contents
Supergene metal deposits form when deeply buried orebodies are exposed at the surface and undergo oxidation, dissolution, and sig- nificant reconcentration of metals. Much of the global economic and scientific interest in these ores stems from their mineralogical diver- sity and advantages for exploitation due to their surficial development and increased grades. Supergene deposits contribute significantly to the world’s supply of metals, such as copper, aluminum, and nickel. They are also increasingly being explored and exploited as alternative sources for “critical metals,” including rare earth elements and strategic metals, which are widely used in technology and low-carbon energy applications. Furthermore, supergene metal deposits provide clues about our past climate and offer an unparalleled opportunity to explore the long-term corrosion behavior and environmental impact of natural and man-made materials. This issue of Elements will highlight some of the most recent advances in the field,including cutting-edge research in economic geology, paleoclimate and geoarcheology studies, environmental geochemistry, geobiology, and corrosion science.
Geological and Economic Significance of Supergene Metal Deposits
Supergene Alteration of Ore Deposits: From Nature to Humans
The Paleoclimatic Signatures of Supergene Metal Deposits
Copper Isotopic Perspectives on Supergene Processes: Implications for the Global Cu Cycle
Predicting Geologic Corrosion with Electrodes
The Geomicrobiology of Supergene Metal Deposits
AHF Analysentechnik
Analytical Instrument Systems, Inc.
Australian Scientific Instruments (ASI)
Bruker Nano
Cambridge University Press
Cameca
Excalibur Mineral Corporation
Geochemist’s Workbench
JEOL
ProtoXRD
Rocks & Minerals
Savillex
Wiley
Zeiss
v11n6 Geomicrobiology and Microbial Geochemistry
Guest editor: Gregory K. Druschel (IUPUI) and Gregory J. Dick (University of Michigan)
Microbes drive the interplay of Earth and life and thus control critical processes in ocean, atmosphere, and terrestrial environments. Indeed, this unseen part of our world has regulated the cycling of key ele- ments throughout geologic time. The field of microbial geochemistry is rapidly advancing our understanding of the chemical, biological, and geologic processes that regulate this cycling. Moreover, with the rapid developments in “omics” techniques (genomics, transcriptomics, and proteomics), a revolution is now underway. New studies are coupling these methods with our geochemical understanding of microbial popu- lations to provide unprecedented insights into how microorganisms shape their surroundings and how geochemistry shapes microbial popu- lations. The authors will show how linking geochemical and microbial information brings understanding of the role of microbes in element cycling in modern and ancient environments.
- Geomicrobiology and Microbial Geochemistry Greg Druschel (IUPUI) and Andreas Kappler (University of Tübingen)
- Principles of Geobiochemistry Eric Boyd (Montana State University) and Everett Shock (University of Arizona)
- Omic Approaches to Microbial Geochemistry Greg Dick and Phyllis Lam (University of Southampton)
- Cryptic Cross-Linkages Among Biogeochemical Cycles: Novel Insights from Reactive Intermediates Brad Tebo (University of Oregon), Colleen Hansel (Woods Hole Oceanographic Institute), and Tim Ferdelman (Max Planck Institute für Microbiologie, Bremen)
- Emerging Biogeochemical Views of Earth’s Ancient Microbial Worlds Tim Lyons (University of California, Riverside), David Fike (Washington University, St. Louis), and Aubrey Zerkle (St. Andrew’s University)
- Emerging Frontiers in Geomicrobiology Alexis Templeton (University of Colorado) and Karim Benzerara (University Pierre et Marie Curie)
- Mineralogy of Mars (February 2015)
- Arc Magmatic Tempos (April 2015)
- Apatite (June 2015)
- Societal and Economic Impacts of Geochemistry (August 2015)
- Supergene Metal Deposits (October 2015)
- Geomicrobiology and Microbial Geochemistry (December 2015)
Download 2015 Thematic Preview