Thermodynamics of Earth Systems
Pascal Richet, Grant S. Henderson, and Daniel R. Neuville – Guest Editors
Table of Contents
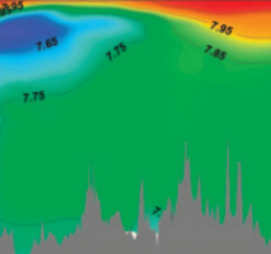
During the past decades, thermodynamics has become an essential tool for under- standing fundamental processes that have determined the structure and evolution of our planet. From the atmosphere to the ocean and sediments, from metamorphic terranes to magmatic provinces, the lower mantle, and the core, this issue of Elements will illustrate how a better understanding of the manner in which free energy depends on temperature, pressure, and chemical composition allows the Earth’s activity to be better deciphered. At a time when climate change has become a major concern, thermodynamic studies of the atmosphere and ocean have not only an academic interest, but also considerable practical importance.
- Thermodynamics: The Oldest Branch of Earth Sciences?
- Thermodynamic Processes in the Moist Atmosphere
- Use of Thermodynamics in Examining the Effects of Ocean Acidification
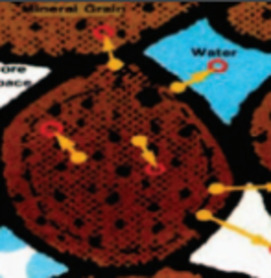
- Water–Rock Interaction Processes Seen through Thermodynamics
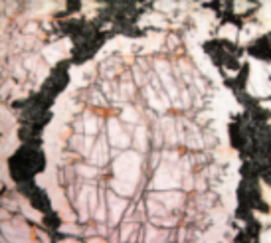
- Using Equilibrium Thermodynamics to Understand Metamorphism and Metamorphic Rocks
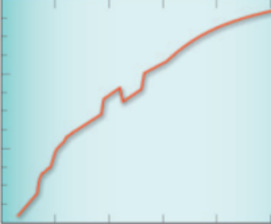
- Thermodynamics of Phase Equilibria in Magma
- Thermodynamic Modeling of the Earth’s Interior
Australian Scientific Instruments (ASI)
Bruker AXS
CrystalMaker
Dakota Matrix
Energy Frontier Research Center– Material Science of Actinides
Excalibur Mineral Corporation
Geological Society of london
ICAM 2011
PERALK-CARB workshop
Rigaku
RockWare
Savillex
Setaram
Solomon Symposium
Thermo Scientific
Wiley-Blackwell
v6n6 SUSTAINABLE REMEDIATION OF SOILS
Guest editor: Mark E. Hodson (University of Reading)
Humanity requires healthy soil in order to flourish. Soil is central to food production, regulation of greenhouse gases, and pro- vision of amenity. But soil is fragile and easily damaged by uninformed management or accidents. One source of damage is contamination with the chemicals that are used to provide the lifestyles to which the developed world has become accus- tomed. Repairing or cleaning up this damage so that soil can again be used for beneficial purposes is a vitally important task. Traditionally, soil “clean up” involved removing the contaminated soil and replacing it with clean soil from else- where. Clearly this is not sustainable. Increasingly researchers and practitioners look to clean up contaminated soil and make it good for reuse, rather than simply discarding it. Mineralogy and geo- chemistry are central to the design and implementation of many of these new approaches.
- The Need for Sustainable Soil Remediation Mark E. Hodson (University of Reading)
- Organic Amendments for Remediation: Putting Waste to Good Use David L. Jones (United States Department of Agriculture) and John R. Healey
- Mineral-Based Amendments for Remediation Peggy A. O’Day (University of California, Merced) and Dimitri Vlassopoulos
- Assisted Phytoextraction: Helping Plants to Help Us Filip M. G. Tack (University of Ghent, Belgium) and Erik Meers
- Bioremediation: Working with Bacteria Blanca Antizar-Ladislao (University of Edinburgh)
- Nanoparticles for Remediation: Solving Big Problems with Little Particles Nicole C. Mueller and Bernd Nowack (EMPA-Swiss Federal Laboratories for Materials Testing and Research)
- Mineral Evolution (February 2010)
- Sulfur (April 2010)
- Fluids in Metamorphism (June 2010)
- Atmospheric Particles (August 2010)
- Thermodynamics of Earth Systems (October 2010)
- Sustainable Remediation of Soil (December 2010)
Download 2010 Thematic Preview